Special Report Offshore production of green hydrogen ①
페이지 정보
작성자 최고관리자 댓글 0건 조회 1,525회 작성일 22-05-16 15:31본문
Green hydrogen as an alternative fuel
The term ‘alternative fuels’ refers to a broad spectrum of non-conventional energy carriers, such as ammonia, methanol, methane, or hydrogen. The increase in demand for alternative fuels as substitutes for conventional fuels can be attributed to the following primary drivers:
• Alternative fuels can help to bring companies in compliance with current statutory regulations, such as carbon emissions requirements.
• Alternative fuel technologies are undergoing rapid development as part of a long-term plan to meet the Paris Agreement’s 2050 climate goals.
• Alternative fuels are more attractive to environmentally conscientious consumers looking for cleaner options that still meet their energy needs.
Direct comparisons between alternative fuels are difficult to make as they are subject to the varied requirements and objectives of the end user. Alternative fuels can have large differences in production techniques, use cases, and storage requirements. Additionally, alternative fuels differ in their technological readiness and maturity. For example, liquefied petroleum gas(LPG) or liquefied natural gas(LNG) fuels are much more widespread and better integrated into the global fuel network than alternative fuels like ammonia and hydrogen due to years of industry experience and historical policy drivers. Hydrogen and its derivatives are seen as viable long-term fuel solutions, but will require significant research and development to reach a comparable level of technological readiness to LPG and LNG.
Hydrogen is produced through chemical reactions that separate it from water or hydrocarbons. In industry, hydrogen is often referred to by different colors to indicate its origins:
• Brown hydrogen, produced via coal gasification or coal carbonization
• Grey hydrogen, produced in the steam reformation reaction using natural gas
• Blue hydrogen, produced in the same manner as grey hydrogen but the emissions are captured resulting in a net-zero carbon footprint from the reformation process
• Green hydrogen, produced from renewable energy sources powering the water electrolysis process with no carbon emissions.
Brown, grey, and blue hydrogen production processes all create their hydrogen as a byproduct of burning fossil fuels. While these processes are possible in offshore environments, there are few compelling reasons to convert hydrocarbons into hydrogen in such an environment. None of these production methods require close proximity to renewable energy sources, and moving those production facilities offshore would only increase capital expenditures relative to comparable onshore projects. For further information on the production and potential use cases of brown, grey, and blue hydrogen fuels in marine applications, see the ABS Sustainability Whitepaper Publication Hydrogen as Marine Fuel.
Unlike brown, grey, and blue hydrogen, green hydrogen is produced by water electrolysis. Electrolysis is the process of passing a direct electric current through an electrolyte, inducing chemical reactions at the electrodes that result in decomposition, or breakdown, of reagents. In the electrolysis of water, water molecules are decomposed into their two elemental components – hydrogen and oxygen – with no additional emissions produced. The electrolysis process is performed by an electrolyzer system.
Green hydrogen production is powered entirely by renewable energy sources. As a result, green hydrogen development relies upon a continued shift toward renewable energy before it can be widely available on a commercial scale. The renewable energy source – in the case of offshore hydrogen production this is predominantly wind power with a small contribution from solar power – will be used to drive the water electrolysis reaction. The produced hydrogen can be exported via ship or pipeline, or can be stored for export at a later date.
Renewable energy leads the development of green hydrogen markets
The production of green hydrogen requires a renewable energy source to provide the input power into the electrolyzer system and any other equipment used in the process. In the case of offshore hydrogen production, the simplest source of renewable energy will come from offshore wind turbines. Other energy sources that may be used in offshore hydrogen production include tidal, wave, and solar energy harvesters. While tidal and wave harnessing technologies could potentially provide the requisite power for the electrolysis reaction, their share of the total offshore renewable power industry is currently orders of magnitude smaller than offshore wind, and they tend to be constructed in harbors or near shorelines. Offshore solar power is also less developed than wind, producing only one-tenth of the power of offshore wind power at facilities typically constructed on sheltered inland ponds and lakes as opposed to at sea.
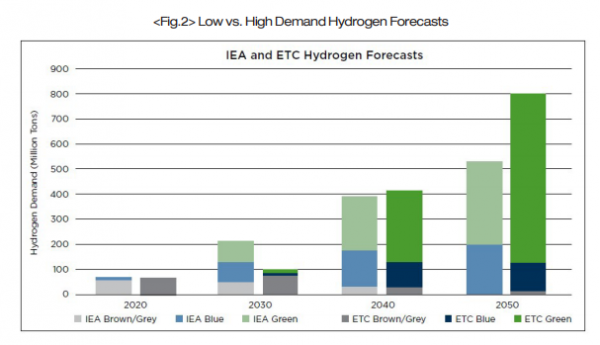
In 2019, the global consumption of hydrogen fuel reached approximately 75 million tons. Of the 75 million tons of hydrogen, only 1.5 million tonnes were green hydrogen. A market analysis performed in April 2021 by the Energy Transitions Commission(ETC) indicated that the demand for hydrogen is expected to increase at a rate of seven to nine percent per year. This would lead to an estimated demand of between 500 and 800 million tons of hydrogen by the year 2050, which would fulfill between 15 and 20 percent of the global energy demand. To reach a production level of 500 million tons of hydrogen in the year 2050, there will need to be 3,000 to 6,000GW of newly installed renewable energy sources devoted to hydrogen production.
In regions that are already heavily invested in developing renewable energy, hydrogen offers a reliable source of fuel during times where renewable energy alone can not meet grid demand. The production of green hydrogen is also a useful outlet for the energy generated during times when renewable power production exceeds the grid demand. In transportation and shipping, hydrogen fuel cells and hydrogen-based combustion engines can offer a reliable fuel source that reduces total emissions.
Reliable sources of renewable electricity for green hydrogen production can be found both onshore and offshore.
Offshore green hydrogen production may be preferred over onshore green hydrogen production when certain conditions exist, such as:
• Unsuitable shore terrain or geography makes construction difficult and/or expensive
• Existing presence of permanent structures or communities
• Local government zoning restrictions targeting any industrial land usage, especially for large-scale facilities
• Possible regional unrest posing a greater security risk to shore-based facilities
• Existing offshore infrastructure suitable for hydrogen production and transport may be cheaper to adapt than constructing new onshore infrastructure
Due to green hydrogen’s reliance on renewable energy sources, initial developments in offshore green hydrogen production will be localized to regions with existing or planned offshore renewable energy systems. The Global Wind Energy Council reported that the United Kingdom(29 percent), the People’s Republic of China(28 percent), Germany(22 percent), the Netherlands(7 percent), and Belgium(6 percent) are responsible for 93 percent of the world’s current offshore wind power production. China has led the world recently in newly installed offshore wind capacity – installing 50 percent of the world’s new offshore wind power capacity in 2021 – and is expected to continue to do so as part of their overall renewable energy plans. Other regions are also beginning to develop offshore wind projects of their own, with countries like the U.S., Japan, and South Korea all expanding from onshore into offshore wind.
Northern Europe and China are two of the more likely regions for immediate development and installation of green hydrogen production facilities, due to the abundance of renewable offshore wind power, existing offshore infrastructure, and government policymaking that’s favorable towards alternative fuels. Other countries like the United States, Saudi Arabia, and Australia are currently investigating and developing large onshore hydrogen production facilities and could potentially expand their scope to include offshore production facilities in coastal areas as well, but are limited by the lack of existing large-scale offshore renewable energy developments. While not an insurmountable challenge, it will take additional investment to develop offshore green hydrogen production facilities in these countries.
Electrolyzers and the electrolysis reaction
The electrolyzer is the core of the green hydrogen production process. The design of the electrolyzer dictates the equipment’s manufacturing cost, physical footprint, supporting equipment requirements, maintenance requirements, and the overall efficiency of hydrogen production. For these reasons, the selection of the electrolyzer design will dictate the design of the entire hydrogen production facility.
All electrolyzers are designed to facilitate the same base electrolysis reaction. As a result, there are several common features between all designs. There is an electrolyte to facilitate ion transfer between the two electrodes(i.e., the anode and cathode) where the chemical reactions occur. The electrodes and the electrolyte form the components of a circuit, where a direct current is supplied by a power source.
Hydroxide is oxidized at the anode, producing water and oxygen gas. At the cathode, water is reduced to produce hydroxide and hydrogen gas. The half-reactions are commonly balanced with a base, but in an acid-balanced reaction the hydrogen is still produced at the cathode and the oxygen is still produced at the anode.
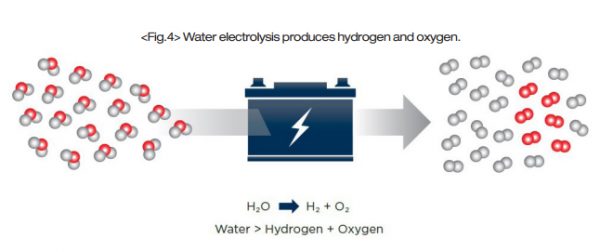
Electrolyzers also have a membrane between the electrodes that permits different molecules to pass through depending on the system design. Other system variables that change between designs include operating temperatures and pressures, electrolyte selection, membrane material, and electrode arrangement.
All electrolyzer designs can be broken down into three distinct levels: the single cell(1st level); the stack(2nd level); and the total system (3rd level). A single cell is the core of the electrolyzer, where the electrochemical process takes place. A stack consists of multiple cells put together and any spacers, seals, or frames required for the connections.
The third level is the total system, or the stack and all additional modules and equipment necessary for the hydrogen production process. This does not include any further gas compression, liquefaction, or storage. The total size of an electrolyzer system depends on the target hydrogen output and the design power input values. Some electrolyzer systems are as small as a refrigerator, while 10MW or larger facilities can occupy spaces over 7,500m2 depending on the exact arrangement of machinery and piping.
Commercial electrolyzers in use today typically require an input power of at least 50kWh(180MJ) to produce 1kg of pure hydrogen. For a single representative wind turbine with 8MW capacity operating at a typical effectiveness of 35 percent powering a modern commercial electrolyzer, 490 metric tons of hydrogen could be produced every year. As of 2021, at least 69 offshore windfarms have a nameplate capacity of over 200MW, and between them all have a total nameplate capacity of over 24,000MW. At 35 percent effectiveness their gross annual energy production could be as high as 70TWh. If the power generated by these large wind farms was leveraged entirely for hydrogen production, just over 1.4 million metric tons of hydrogen could be produced every year.
Electrolyzer disign comparison
Currently there are three commercially viable designs of electrolyzers being considered for use in hydrogen production: proton exchange membrane(also known as polymer electrolyte membrane, or PEM) electrolyzers; alkaline electrolyzers; and solid oxide electrolyzers.
Solid oxide electrolyzers operate using a solid ceramic membrane that requires operating temperatures upwards of 700°C to function. This elevated temperature can be efficiently achieved for hydrogen production processes where heat is already being produced, such as in nuclear reactors. When the energy comes from renewable sources operating at near ambient temperatures however, any additional heating of the water reduces the overall efficiency of the hydrogen production process. Solid oxide electrolyzers do not require any precious metals in their construction but do have lower longevity when compared to other electrolyzer designs.
Alkaline electrolyzers use a liquid alkaline solution of either potassium hydroxide(KOH) or sodium hydroxide(NaOH) to facilitate the electrolysis reaction. The working temperature for alkaline electrolyzers is between 60°C and 90°C, and the working pressure is between 1 bar and 30 bar. Alkaline electrolyzers can be either unipolar or bipolar in design. Unipolar designs, also known as monopolar or tank designs, have their electrodes suspended in parallel in alternating tanks separated by thin membranes that allow for the transfer of ions, but restrict the movement of the produced gases. Bipolar designs position the electrodes very close to each other, separated by a thin non-conductive membrane. Unipolar designs have the advantage of being cheaper and easier to build and maintain but are typically less efficient than bipolar designs. Alkaline electrolyzers operate best near their design loads, and they experience a drop in efficiency when operating under lower loads. Both of the designs for alkaline electrolyzers are more durable and contain fewer expensive rare earth metals than PEM and solid oxide electrolyzers.
PEM electrolyzers are similar to bipolar alkaline electrolyzers, but don’t require an electrolytic solution to function. Instead, PEM electrolyzers have a thin, solid electrolyte membrane that permits hydrogen ions to pass through. PEM electrolyzers typically operate at higher current densities and higher pressures than their alkaline counterparts. The increased current density enables a more rapid system response to fluctuations in energy input, which can be a great benefit when working with intermittent renewable energy sources. They operate at temperatures between 50°C and 80°C, but at higher pressures than alkaline electrolyzers. Typical PEM electrolyzers are constructed using more rare earth metals than alkaline electrolyzers and require more precise construction techniques for their catalysts, which makes them more expensive to produce and maintain.
Each electrolyzer design has its own unique benefits and drawbacks, the selection of any particular design will influence the design of the complete facility and vice versa. They each require different pre-processing techniques for the supplied water, have different operating conditions, and have different maintenance requirements. In order to understand where each electrolyzer could be optimal, the complete hydrogen production facility must be examined.
Hydrogen production facilities
Even though the offshore hydrogen industry doesn’t have a long history, decades of offshore experience with both the oil and gas industry and the wind power industry provide many useful parallels to draw from. The challenges of adapting land-based hydrogen production facilities to offshore applications are numerous and vary in complexity.
Some challenges associated with marinization are already well understood though, as the hydrocarbon and wind industries already addressed them when they began their own transitions from onshore to offshore applications:
• Large-scale hydrogen production facilities on land take up considerable space, and at the 100+MW scale they have a significant footprint. Finding the optimal size of a production facility to maximize limited space will be an important factor in initial planning and design of the facility, or when refitting an existing facility for hydrogen production.
• Environmental factors vary greatly depending on the region, and no two locations are the same. When designing the facility, typical and extreme wave and wind conditions at the site can drive decisions regarding the size, shape, and mooring arrangements when applicable.
• The proximity to renewable energy sources and any existing hydrogen infrastructure is also important to consider.
For example, a facility close to a windfarm may reduce any power transmission losses and cable length but could require longer pipelines for exporting the hydrogen to shore.
In response to these and other challenges, there have been several proposed designs for offshore green hydrogen production facilities. These designs can be broadly categorized by the location of the electrolyzer and other associated core systems relative to the renewable energy source:
1. Directly incorporated into the structure of the renewable energy source(such as a wind turbine tower)
2. Not directly incorporated into the structure of the renewable energy source and located above water on a platform
3. Not directly incorporated into the structure of the renewable energy source and located on the seabed
The first option has only been considered with respect to offshore wind farms and would have the electrolyzer directly integrated into the turbine structure. The electricity being produced is directed to the electrolyzer, seawater pumps, and any other equipment as necessary. The electrolyzer would then produce hydrogen and export it via delivery pipelines to a collection manifold located on either a platform or at the seabed, where the gas can be compressed to the desired pressure and exported to shore via a larger supply pipeline. This approach becomes more viable as the nominal power supplied by the wind turbines increases, since more powerful electrolyzers could be installed. Additional modifications may also be necessary for the wind turbine to accommodate the additional machinery on deck or within the structure. Existing wind farms will need to undergo extensive infrastructure modifications if this option is chosen, as pipelines would need to be laid and each turbine structure could require modifications.
The second option generally refers to the use of a floating or fixed platform with one centralized electrolyzer system. This design would require fewer changes to incorporate into existing renewable energy infrastructure.
After incorporating the production platform into the system delivering power to shore, the platform is largely independent. The supplied power will be used to run the water supply pumps, electrolyzers, heat exchangers, compressors, and any other equipment as needed on the platform. As the hydrogen is produced it is compressed, and either immediately exported or undergoes additional compression and refrigeration for storage as either a gas or a liquid. It may be possible to retrofit existing offshore assets like platforms and pipelines to reduce the capital expenditure of a project.
The third option would place the entire centralized electrolyzer system on the seabed. This could be a viable alternative in areas where surface-based operations are not possible or desirable. This design reduces some of the power lost to pumping electrolyzer feed water in surface designs and negates most of the weather-related effects that surface designs may face, but does add complexity and additional construction costs to the project. Maintenance of any subsea system is more expensive to perform than a comparable surface system, so electrolyzers would need to be designed for longer maintenance intervals with more reliable catalysts and electrodes.
When considering the overall design of the production facility, there are some considerations that are generally applicable to any project. For example, green hydrogen relies on renewable energy sources to power the entire process. In the event that insufficient power is being supplied to the facility, the production system needs to be able to either safely shut down or operate at a reduced rate. For electrolyzer stacks, this could be done by taking some cells offline. Some hydrogen could be stored permanently onsite and used in conjunction with fuel cells as a backup power supply to ensure essential systems remain functioning until renewable power production returns to its normal rate.
Another factor to consider is how the facility will be crewed, if at all. While platform designs could dedicate some space to crew accommodations, subsea designs are only accessible to remotely operated vehicles or divers, depending upon the water depth. For decentralized designs, electrolyzers could be monitored and operated remotely from shore, or from a collection manifold if it’s located on a platform. For additional information on autonomous and remote control system design, see the ABS Guide for Autonomous and Remote Control Functions.
As the demand for offshore production of green hydrogen grows, the ability to expand production capacity is also important. Choosing designs that are easily expandable could be more cost effective in the long run, especially in regions with large amounts of available renewable energy and forecasts for high hydrogen demand. Because hydrogen can also serve as a feedstock for producing other chemicals, like ammonia, designs might also consider additional systems for generating, storing, and exporting other chemicals in the future.
※ Will continue next month.
- 이전글Additive Manufacturing enters the maritime mainstream 22.05.16
- 다음글댄포스(Danfoss), 새로운 VACON® 1000 고압 드라이브 출시 22.04.15